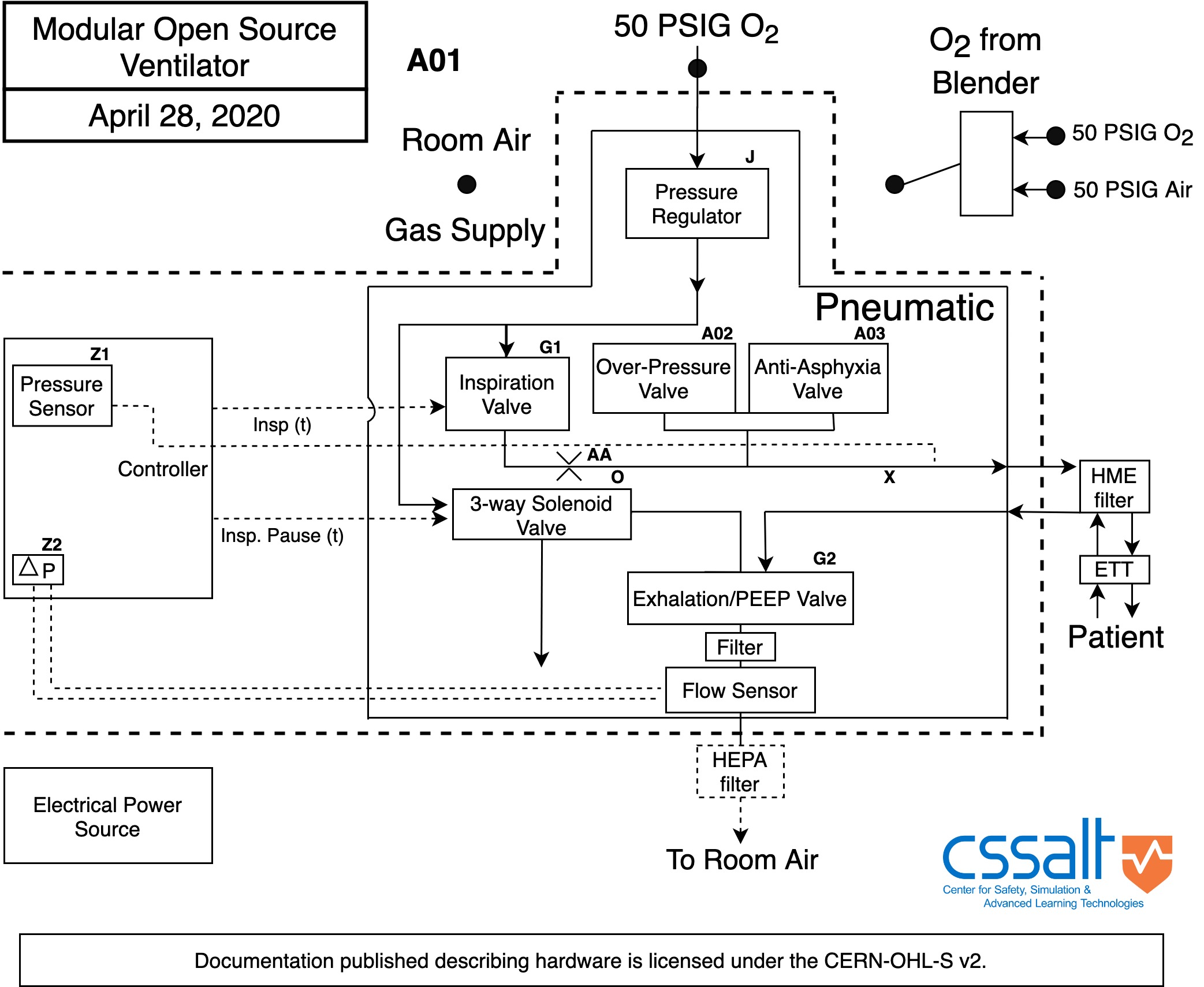
Ventilator Engineering Specifications
(Preliminary and subject to change as needed to meet end-user needs)
Specification Documents
Specifications
| Range | Accuracy | Settings | |
|---|---|---|---|
| Volume Controlled Intermittent Mechanical Ventilation | –– | –– | –– |
| Tidal volume (TV) | 250 – 800 ml | ± 25 ml | Increments of 50 ml |
| Respiratory rate (RR) | 10 – 30 bpm | ± 0.5 bpm | Increments of 2 |
| I:E ratio | 1:1 or 1:2 (1:3 optional) | – | 2 fixed settings and 1 optional setting |
| PEEP valve | 5 – 25 cm H2O; Default setting 5 cm H2O | ± 1 cm H2O | Can either be adjustable in 5 cmH2O increments or continuously adjustable |
| Air and O2 supplied at 50 ± 5 psig (345 kPa) | –– | –– | –– |
| FiO2 | 0.21 or 1.0 | –– | –– |
| Anti-asphyxia valve | Opens at –3 cm H2O | ± 1 cm H2O | –– |
| Over-Pressure Valve | Opens at 40 default, adjust up to 60- cm H2O | ± 5 cm H2O | Manually controlled |
| –– | –– | –– | |
| Optional | –– | –– | –– |
| Plateau pressure | Limited to 35 cm H2O | –– | Controlled in software |
| Peak pressure | < 2 cm H2O + Plateau | –– | Controlled in software |
| Inspiratory pause | 25% inspiratory time | –– | –– |
| Spontaneous breathing | –– | –– | Ventilation mode |
| In-built blender | 0.21 – 1.0 FiO2 | ± 0.05 (+/- 5%) | 3 fixed FiO2 settings: 0.21, 0.5, 1.0; Variable between 30% and 100% in 10% steps preferred |
| Oxygen sensor | 0.21 – 1.0 FiO2 | ± 0.05 FiO2 |
Sensors (optional – not in base design):
Airway pressure sensor
- Connected at 22mm inspiratory port connection
- Capable of measurement ranges of -20 cmH2O to 60 cmH2O (rated for 120 cmH2O over-pressurization)
- at least 1.0 cmH2O resolution
- Used for:
- disconnect and overpressure alarms
- displaying PEEP, peak and plateau airway pressure with waveform (waveform optional)
- aborting inspiration if High inspiratory airway pressure reached
Flow Sensor
- Connected at expiratory port
- Capable of measurement during maximum conditions: max TV 800 ml, RR 30, IE 1:2
- Max flowrate at least 1200 mL/s (72 LPM)
- Accuracy +/- 40 mL/s
- Max flow resistance 2.2 cmH2O at 60 LPM
- Used for:
- Measuring TV via integration over time (measured TV accurate to +/- 25 ml nominal, ideally +/- 10 ml)
- Low/High TV alarms
- Must be able to withstand airway pressures (rated for 120 cmH2O over-pressurization)
User Interface:
- Display
- 20×4 LCD display or equivalent for base design, non-base design supports touchscreen
- Capable of continuously displaying:
- User settings for TV, RR, I:E
- Ventilator output for TV, PEEP, airway pressure (Paw)
- Optional (FiO2, ventilation mode, EtCO2, ventilator output for RR)
- Alarms
- Adjustable variables
- RR,
- I:E,
- TV,
- Alarm limits
Alarms:
- Patient Disconnect
- Condition: pressure does not rise above 3 cmH2O during inspiration
- Audible alarm and UI text “LOW AIRWAY PRESSURE” [16 Char: LOW AIRWAY PRES! (16 characters)]
- High inspiratory airway pressure
- Condition: airway pressure above alarm setting during inspiration
- Adjustable range: 15- 60 cmH2O, increments of 5 cmH2O
- Default setting: 35 cmH2O
- Audible alarm and UI text “OVER-PRESSURE ALARM” [16 char: OVER PRES ALARM! (16 characters)]
- Controller feedback:
- Inspiratory solenoid valve closes to stop inspiratory flow
- Exhalation valve opens (if controlled by solenoid)
- TV High/Low
- Adjustable range: 200-1000 ml, increments of 50 ml
- Default low alarm setting: 200 ml; Default high alarm setting: 1000 ml
- Audible alarm and UI text:
- “TV HIGH” Condition: measured TV exceeds high TV alarm setting
- “SET TV NOT DELIVERED” Condition: measured TV less than low TV alarm setting [16 Char: SET TV NOT DELIV]
- “LOW VT, IT SHORTENED” Condition: Inspiration Time (IT) is shortened by reaching high inspiratory airway pressure limit and measured TV less than low TV alarm setting [16 Char: LOW VT/IT SHORT]
- MV (Minute ventilation) High/Low (Optional):
- Adjustable range: 2 L- 15 L, increments of 1 L
- Default low setting: 4 L; Default high setting: 8 L
- “MV HIGH” Condition: MV greater than high MV alarm setting
- “MV LOW” Condition: MV less than low MV alarm setting
- Gas pipeline failure (Optional)
- Condition: no supply pressure detected
- Audible alarm and UI text “GAS SUPPLY FAILURE” [16 Char: GAS SUPPLY FAIL! (16 char)]
- Electricity supply failure (Optional)
- Condition: ventilator unplugged from wall mains
- Audible alarm and UI text “ELECTRIC SUPPLY FAIL” [16 Char: ELECT SUPP FAIL! (16 char)]
- Machine switched off when in mandatory ventilation mode (“Rapidly manufactured ventilator system specification”, 2020)
- 16 Char: “VENT TURNED OFF!”
- If multiple alarms simultaneously present and display has one line of text for alarms, display line will toggle through all alarms at one second intervals
Incoming gas supply:
- Must connect to wall pipeline or gas blender
- Male Oxygen DISS connector (from hose)
- (Optional) All gas connectors and hoses must use standard non-interchangeable connectors and be color coded according to current standards (“Rapidly manufactured ventilator system specification”, 2020).
- (Optional) Can incorporate a backup oxygen cylinder connected via either Schrader valve or Pin Index System (“Rapidly manufactured ventilator system specification”, 2020).
Electrical supply:
- Ability to connect to 120-240V mains
- Must have 30 minutes backup battery in case of mains electricity failure
- Optionally hot swappable batteries so that it can be run on battery supply for an extended period, for example, 2 hours for within hospital transfer (“Rapidly manufactured ventilator system specification”, 2020).
- Must avoid harmful RF or EM emissions that could interfere with other critical machinery (“Rapidly manufactured ventilator system specification”, 2020).
Gas supply to patient:
Patient breathing system connections- the ventilator must present 22mm outside diameter (OD) ‘male’ standard connectors for connection to user supplied 22mm ‘female’ connectors on the breathing system (“Rapidly manufactured ventilator system specification”, 2020).
All elements in the gas pathway must meet biological safety and oxygen safety standards, especially to minimize risk of fire or contamination of the patient’s airway (“Rapidly manufactured ventilator system specification”, 2020).
Infection Control:
- All parts in contact with pathogens must be either disposable or able to be decontaminated between patients (“Rapidly manufactured ventilator system specification”, 2020).
- All external surfaces must be cleanable in the likely event that they get respiratory secretions or blood splatter on them. Cleaning would be by healthcare workers manually wiping using an approved surface wipe with disinfectant or cloths and approved surface cleaning liquid (“Rapidly manufactured ventilator system specification”, 2020).
- Ability to add in line, replaceable HEPA and HME filters to inspiratory circuit.
Miscellaneous:
- Exhalation valve flow resistance: no more than 1.5 cmH2O at 60LPM gas flow
- Over-Pressure Valve flow resistance: no more than 1.5 cmH2O at 60LPM gas flow
- Anti-asphyxia valve flow resistance: no more than 1.5 cmH2O at 60LPM gas flow
- Must be able to deliver 800ml TV 30BPM I:E 1:2 at a constant flow throughout entire inspiration cycle
- It must have 100% duty cycle (100% up time) for up to 14 days. Optionally it can be used beyond 14 days, the expected durability must be specified (“Rapidly manufactured ventilator system specification”, 2020).
- Can be floor standing (“Rapidly manufactured ventilator system specification”, 2020).
- Ideally small and light enough to mount on patient bed and orientation-independent functioning (“Rapidly manufactured ventilator system specification”, 2020).
- Should be as robust as possible. For example, it may be dropped from bed height to floor (“Rapidly manufactured ventilator system specification”, 2020).
- It must be intuitive to use for qualified medical personnel, but these may not be specialists in ventilator use (“Rapidly manufactured ventilator system specification”, 2020):
- must not require more than 30 minutes training for a doctor with some experience of ventilator use (“Rapidly manufactured ventilator system specification”, 2020)
- Must include instructions for use (“Rapidly manufactured ventilator system specification”, 2020)
- Must have transparent design, supply chain, manufacture and testing processes that are of sufficient quality to enable regulatory officials to deem appropriate for usage in exceptional circumstances (“Rapidly manufactured ventilator system specification”, 2020).
- Must not be excessively cumbersome so that it would impede hospital operations or prevent easy movement within hospital premises (“Rapidly manufactured ventilator system specification”, 2020).
- Avoidance of hot spots (overheating components).
References:
Rapidly manufactured ventilator system specification. (2020, March 20). Retrieved March 24, 2020, from https://www.gov.uk/government/publications/coronavirus-covid-19-ventilator-supply-specification/rapidly-manufactured-ventilator-system-specification